Abstract
Background:
Acute myeloid leukemia (AML) is a myeloid neoplasm of heterogeneous biological and clinical characteristics. Despite advances in molecular diagnostics and novel therapeutics, outcomes of AML patients remain dismal. In an attempt to improve patient outcomes, several phase I trials of novel agents are undertaken each year. In this study, we aimed to analyze the enrollment patterns and response rates of AML patients among phase I clinical trials that were reported over a period of 6 years.
Methods:
A pooled retrospective analysis was conducted on phase I clinical trials of AML published from 2011-2016. This cohort was subdivided into two groups based on year of publication: Group 1 included trials from 2011-2013 and Group 2 from 2014-2016. Data abstracted included sample size, year of publication, median number of prior therapies, single agent or combination therapy, and if the trial was sponsored by industry. Primary outcome measure was overall response rate (ORR) as it was the most frequently reported endpoint. Chi-square test was used to assess the association between year of diagnosis and other variables. Multivariable analysis was adopted to analyze the factors associated with improved/worse ORR.
Results:
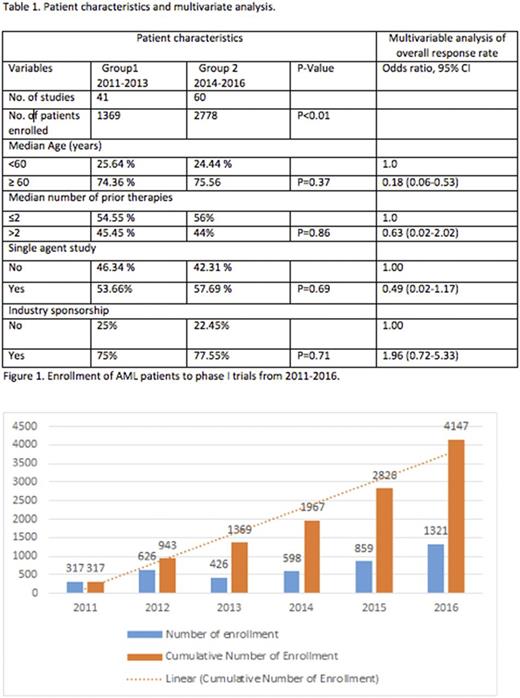
A total of 5516 adult patients, enrolled in 101 studies were included in the study. Of these patients, 1,369 (33.0%) were included in trials from 2011-2013 and 2,778 (67.0%) from 2014-2016. There were more patients enrolled from year 2014-2016 compared to 2011-2013 (p<0.01) (Figure 1). Trials from 2014-2016 included higher proportion of elderly patients (≥ 60 years) as compared to 2011-2013, but was statistically insignificant (p=0.37) (Table 1). Median number of prior therapies also did not appear have a significant difference between two enrollment periods (p=0.86). On multivariable analysis, the only factor associated with an improved ORR was age ≤60 years. Other factors that showed a statistically insignificant association with lower ORR were median number of prior therapies ≥ 2 as compared to <2, and trials utilizing single agent therapy as compared to combination therapy (Table 1). When comparing the two time periods, the ORR did not show a statistically significant difference (HR for 2014-2016 0.59, 95% CI 0.26-1.34).
Discussion:
Our study showed an increased enrollment of AML patients to phase I clinical trials over the last 3 years (2014-2016) as compared to the time period before that. However, the ORR has not improved significantly over the same time period. Most of the trials included heterogeneous patient population and very few of them had pre-specified correlative studies to identify subgroups of patients that respond to therapy. Our study highlights the challenges in identifying effective therapies in AML, and calls for a critical appraisal of clinical trial designs to optimize patient outcomes.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal